When you saw the topic you might have thought "That's easy, I will use thermometers", but then what does the thermometer actually show? What is temperature? We all studied in school that it is a measure of hotness of a body. Then, what is hotness? Now that is difficult to explain so you may try to define Temperature as a measure of energy. Let us see how the Temperature is actually defined and how Thermometers work. Alternate Murphy's Law states-"Whatever that can happen, will happen". This is similar to Infinite Monkey Theorem that says that a monkey striking random keys on a typewriter forever will eventually type the complete works of William Shakespeare.
Ya, as crazy as it sounds this event DOES have a finite probability of occurring and is theoretically possible. Similarly, we do that in Statistical Mechanics and as the name suggests it deals with mechanics in terms of probabilities and Statistics. Purely by common sense of probability, we can build up basics of Statistical Mechanics. Thus whenever the probability of occurence of a state of a system is maximum you will most likely find that as the equillibrium state of the system. Along the same lines, we define "Thermal Equilibrium" between bodies as the state with maximum probability for particles of systems to be in that state, which with some mathematics translates to -
... where,...
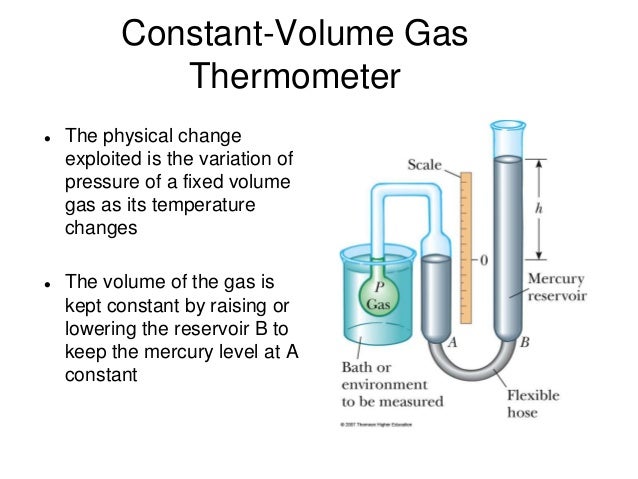
Thus there has to be a quantity which is equal between two bodies at thermal equilibrium. Now we define Temperature, ... as a quantity which is equal at thermal equilibrium with ... Now, this can be any “Temperature parameter” i.e it can be the length of mercury column or resistance in electric thermometers etc. But we consider the above ... to be the absolute temperature and same one as in the Ideal gas equation ,being proportional to Pressure, ... at constant volume of gas. But, it is set according to our choice in accordance with above constraints, with the numerical value being completely our choice(i.e it can be scaled up or down from a certain agreeing value).National Bureau of Standards used Triple Point of Water , the point where all three phases of water coexist. Here I used "the" point to emphasize that ther is only one unique pressure and temperature when this happens. This was set to be
...
Now the obvious question is why "273.16"? Why not 300 or 100 or some other simple value. This choice of value was motivated by desire to keep all the previous temperature observation(.Here we can see the similarities between Kelvin scale) consistent with the new system, else one has unnecessary work converting from one units to another. Another question would be what was wrong with ... since it is also equal at thermal equilibrium. But the essential difference between the two is that while this decreases as Energy increases, Temperature increases which are what we usually observe with pressure and volume and hence we take T as temperature.
From here on we can use above mentioned relation between... and ... with constant volume Thermometers to get Temperatures of other bodies/points using
...
We may also use constant Pressure thermometers which is our normal Mercury Thermometers in similar way find Temperature based on expansion or compression of gas colums in thermometers. Now we also have to set... in accordance with these values. This can be easily done using Ideal Gas equation ... Knowing pressure, volume and temperature of 1 mole of gas(Avogadro number ... ) we can determine ... which turns out to be -
...
This was called ... ,in honor of great Austrian Physicist Ludwig Boltzmann. In this way ... was determined. All this was adopted by international conventions in 1954 as close to historical values as possible.
Ya, as crazy as it sounds this event DOES have a finite probability of occurring and is theoretically possible. Similarly, we do that in Statistical Mechanics and as the name suggests it deals with mechanics in terms of probabilities and Statistics. Purely by common sense of probability, we can build up basics of Statistical Mechanics. Thus whenever the probability of occurence of a state of a system is maximum you will most likely find that as the equillibrium state of the system. Along the same lines, we define "Thermal Equilibrium" between bodies as the state with maximum probability for particles of systems to be in that state, which with some mathematics translates to -
From here on we can use above mentioned relation between
We may also use constant Pressure thermometers which is our normal Mercury Thermometers in similar way find Temperature based on expansion or compression of gas colums in thermometers. Now we also have to set

Comments
Post a Comment